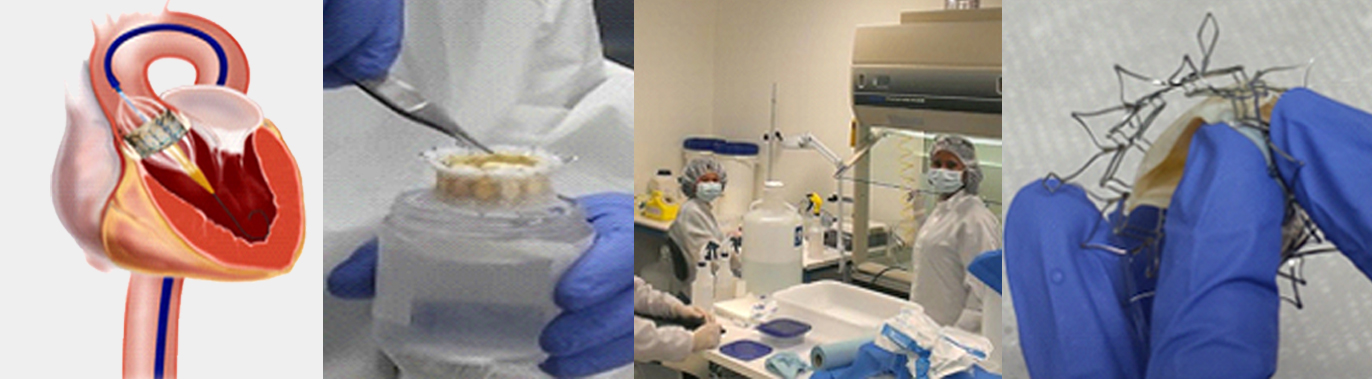
A startup medical device company engaged us in developing a Transcatheter Mitral Valve (TMVR) based on their Intellectual Property. This was an end to end project; our client lacked the in-house resources like clean rooms, wet and dry labs, testing equipment and other resources…
This is a startup medical device company developing the porcine TAVR valve. In this project we helped the client setup their chemical solutions (wet) preparation lab for cross-linking the tissue. We helped the client in successfully setting up the entire wet lab…
Startup medical device company specializing in TAVR product. Client wanted to submit for FDA – IDE, Canadian Medical licensing, and CE marking for its product. One of the requirements for these submissions is to demonstrate biocompatibility of the device.
This is a startup medical device company engaged us in developing a Transcatheter Mitral Valve (TMVR). Client lacked the resources and the knowledge to successfully assemble the tissue to the valve. This was a challenging project due to client’s unique valve design..
Israel startup medical device company developing class III devices for cardio vascular therapies. The client wanted to write a rationale for viral inactivation studies based on literature data and historical clinical use if the device for First in human (FIH) trail of the class III medical device. The client was a start-up company with limited […]
Ireland division of publicly listed medical device company. The client wanted to write a rationale for biocompatibility for their Transcatheter delivery system based on their previous generation of the same device. BridgeMed solutions consultant analyzed the project, went through the previous biocompatibility studies, and wrote rationale using previous studies, literature data and historical use of […]
A startup company wanted to perform liquid chemical sterilization validation as per ISO 14160 for their class III medical devices. BridgeMed solutions consultant took over this project, wrote protocol in compliance with ISO 14160, and identified the labs to perform the studies, coordinated and conducted the studies necessary for this sterilization validation. Finally analyzed all […]
A publicly listed world’s largest medical technology company with over $ 57 Billion in market cap offering wide varieties of innovative therapies. Client operates in over 140 countries offering medical therapies in Cardiac, Vascular, Diabetic, Neurological and Musculoskeletal treatments. Client was manufacturing multiple structural heart valve products, each with different chemical treatments specifications for viral […]
Startup medical device companies in business of design develop and market innovative catheter-based technologies for the treatment of certain structural heart defects. Medical Device Company was in the process of obtaining CE mark for a class III medical device. Added a new manufacturing facility (with clean rooms etc.) and expecting a surveillance audit from a […]
A publicly listed medical technology company is a leading global provider of innovative devices for the treatment of peripheral vascular disease. Also develop, manufacture, and market disposable and implantable vascular devices to address the needs of vascular surgeons. The client did not have in-house expertise to prepare the documentation is necessary to submit for EDQM […]
A publicly listed world’s largest medical technology company with over $ 50 Billion in market cap offering wide varieties of innovative therapies. Client operates in over 140 countries offering medical therapies in Cardiac, Vascular, Diabetic, Neurological and Musculoskeletal treatments. The client was consolidating three structural heart valve manufacturing sites into one site. This activity required […]