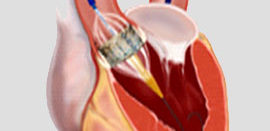
Startup medical device company specializing in TAVR product. Client wanted to submit for FDA – IDE, Canadian Medical licensing, and CE marking for its product. One of the requirements for these submissions is to demonstrate biocompatibility of the device.